
Viral Vectors: The Backbone of Cell and Gene Therapy
Cell and gene therapies (CGTs) have advanced from conceptual promise to clinical reality, with dozens of approved products and thousands more in development. A unifying feature across this pipeline is the viral vector: an engineered virus optimized to deliver therapeutic payloads with high efficiency. Far from being an ancillary tool, vectors are often the rate-limiting step in moving programs forward, both scientifically and operationally.
Viral-Based Gene Therapies
While the cell and gene therapy landscape has been changing dramatically over the past couple years, as of July 2025, there were nearly 4,500 therapies in development between preclinical to pre-registration. Of those, 2,210 (49%) were gene therapies including CAR-T cell therapies. Of these, viral vectors continue to be used most prominently to deliver genetic material to target cells.

Figure 1. Pipeline of gene, cell, and RNA therapies. (Source: Gene, Cell, & RNA Therapy Landscape Report Q2 2025)
Viral Vectors in Practice
Most clinically advanced platforms rely on AAV and lentiviral vectors, each with advantages and trade-offs:
- AAV offers episomal persistence, relatively low immunogenicity, and tropism for non-dividing cells, making it the vector of choice for in vivo gene delivery. Its constraints are well known: a modest ~4.7 kb payload capacity, serotype-specific biology, and the challenge of large-scale, high-yield production.
- Lentivirus integrates into dividing and non-dividing cells, enabling durable modification of T cells and hematopoietic stem cells. It underpins the vast majority of CAR-T programs. But LVV production is inherently complex, with critical steps in plasmid design, transfection efficiency, and downstream purification all impacting infectious titer and functional potency
Other systems, including γ-retrovirus, adenovirus, and non-viral alternatives (LNPs, exosomes), have roles in specific applications, but the industry’s near-term bottlenecks are squarely in AAV and LVV scale-up.
![southwest illustration [Converted]](https://blog.landmarkbio.com/hs-fs/hubfs/southwest%20illustration%20%5BConverted%5D.png?width=1251&height=581&name=southwest%20illustration%20%5BConverted%5D.png) Figure 2. Diverse modalities within cell and gene therapy.
Figure 2. Diverse modalities within cell and gene therapy.
Market Momentum Meets Manufacturing Constraints
The field continues to grow, and CAR-T alone accounts for 55% of gene-modified cell therapy programs, the majority of which depend on lentivirus.
 Figure 3. CAR-Ts continue to dominate the gene therapy breakdown. (Source: Gene, Cell, & RNA Therapy Landscape Report Q2 2025)
Figure 3. CAR-Ts continue to dominate the gene therapy breakdown. (Source: Gene, Cell, & RNA Therapy Landscape Report Q2 2025)
The surge in demand has exposed systemic weaknesses:
- Process variability. Academic-originated processes often lack the rigor needed to transition efficiently into GMP.
- Regulatory pressure. Agencies expect increasingly sophisticated analytical packages (e.g., ddPCR-based titers, potency assays in permissive cells) earlier in development.
- Time-to-clinic. Small and mid-sized biotechs, under pressure from investors and competition, cannot afford 12–18-month delays for tech transfer.
Landmark Bio’s Viral Vector Platforms
Landmark Bio was established to address these exact bottlenecks. Our modular facility in the Greater Boston hub provides end-to-end support for viral vector development and GMP manufacturing, with capabilities spanning discovery through clinical supply.

Figure 4. Mirrored process/analytical development and cGMP enables streamlined scale-up for both AAV and LVV programs.
Upstream Systems
- HEK293 suspension cultures scaled from 10L to 200L.
- Triple-transfection AAV systems and third-generation LVV plasmid systems.
- Bioreactors designed for reproducibility and scalability.

Figure 5. Landmark Bio’s scalable upstream workflow for both LVV and AAV.
Downstream and Formulation
- Clarification via depth filtration with recovery rates matching or exceeding literature.
- Capture and polishing chromatography.
- Concentration and formulation using using tangential flow filtration.
- Optional viral filtration and fully integrated fill/finish.
 Figure 6. Landmark Bio’s scalable downstream workflow for both LVV and AAV (titer data shown is LVV).
Figure 6. Landmark Bio’s scalable downstream workflow for both LVV and AAV (titer data shown is LVV).
Full Analytical Workflow
- Quantitative genomic titers via qPCR and ddPCR.
- Infectious and transducing titers to evaluate potency.
- P:I ratios ≤ 500, ensuring consistent product quality
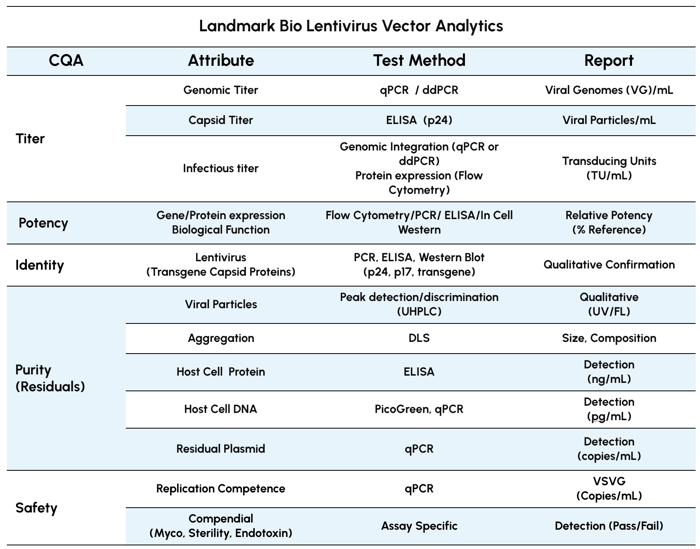
Table 1. Thorough QC testing mirrored in both process development and GMP workflows.
Looking Ahead
As CGTs expand into more prevalent indications—oncology, cardiovascular, and metabolic disorders—the scale and consistency of vector supply will determine who reaches patients first. The industry cannot afford bottlenecks at the very foundation of therapeutic development.
Landmark Bio’s viral vector platforms are designed to translate discovery into clinic-ready programs quickly and reliably, while maintaining the analytical depth and regulatory foresight required for long-term success.
Conclusion
Viral vectors remain indispensable in CGT development, but their complexity makes them the single most critical chokepoint in the pipeline. For companies pushing the boundaries of genetic medicine, aligning with a partner who combines technical depth, modality breadth, and execution speed is no longer optional — it’s strategic.
At Landmark Bio, we are that partner.
📩 Connect with us to learn how our viral vector capabilities can accelerate your program.


